Solving the Doping Problem: Physicists Discover New Ways to Improve Organic Semiconductors
Instruments used to measure the properties of semiconductors. Credit: Dr Martin Statzs, Sirringhaus Lab
Scientists have developed organic semiconductors by achieving the removal of strong electrons and using the property of the unbalanced state, which is an opportunity to increase the efficiency of the thermoelectric device.
Cavendish physicists have discovered two new ways to improve organic semiconductors. They found a way to remove more electrons than was previously possible and used an unexpected property in an environment known as an unstable state, enhancing its performance for use of electronic devices.
“We really wanted to hit the nail on the head and find out what happens when you use polymer semiconductors a lot,” said Dr. Dionisius Tjhe, Postdoctoral Research Associate at Cavendish Laboratory. Doping is the process of removing or adding electrons to a semiconductor, increasing its ability to conduct electricity.
In a recent paper published in Natural ThingsTje and his colleagues explain how these novel observations can help improve the performance of doped semiconductors.
Power groups with unexpected levels of doping
Electrons in a solid are organized into energy groups. The highest energy group, known as the valence group, controls many important physical properties such as electrical conductivity and chemical bonding. Doping in organic semiconductors is achieved by removing a small fraction of electrons from the valence band. Holes, the absence of electrons, can flow and conduct electricity.
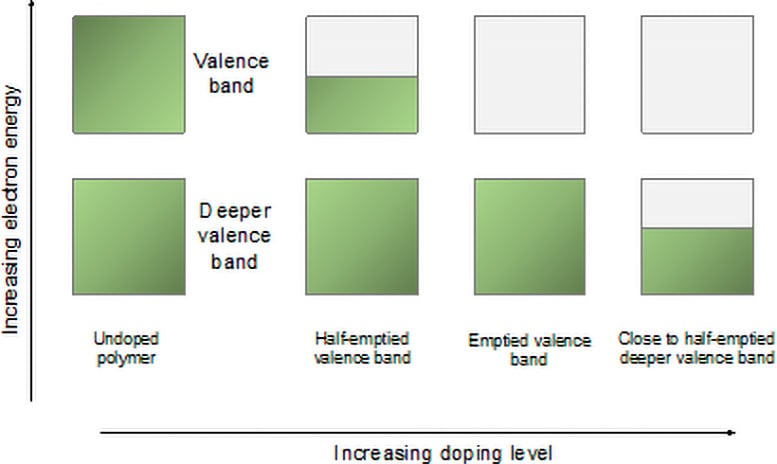
Removal of valences and deep valence groups by doping. Credit: Sirringhaus Lab
“Typically, only ten to twenty percent of the electrons in the organic semiconductor’s valence group are removed, which is much higher than the parts per million common in silicon semiconductors,” said Tjhe. . “In two of the polymers we studied, we were able to remove the valence band completely. Even more amazingly, in one of these materials, we could go further and remove the electrons from the bottom class. It would be the first time that was achieved!”
Interestingly, the conductivity is much greater in the deep valence band, compared to the upper one. “The hope is that transport transport in deep energy levels can eventually lead to very powerful, thermoelectric devices. These convert heat into electricity,” said Dr Xinglong Ren, Postdoctoral Research Associate at Cavendish Laboratory and author-‘ He is also the first person in education. “By finding high-efficiency materials, we can convert a lot of waste heat into electricity and make it a more efficient source of energy.”
Why was this noticed in the information?
Although the researchers believe that the removal of the valence group is possible with other materials, this effect may be more easily seen in polymers. “We think that the way the energy groups are organized in our polymer, as well as the unstable nature of the polymer chains allows us to do this,” said Tjhe. “On the other hand, other semiconductors, such as silicon, probably do not have the opportunity to hold these effects, since it is very difficult to shed the valence group in these materials. Understanding how to produce this effect in devices ling is the next important step. It’s an exciting time for us.”
Is there another way to increase thermoelectric efficiency?
Doping leads to an increase in holes, but also increases the number of ions, which reduces the energy. Fortunately, researchers can control the number of holes, without affecting the number of ions, by using an electrode known as a field effect gate.
“Using a field effect gate, we found that we could change the hole size, and this led to very different results,” explains Dr Ian Jacobs, Royal Society University Research Fellow at Cavendish Laboratory. “Conductivity is generally proportional to the number of holes, it increases as the number of holes increases, and decreases as they are removed. This is seen when we change the number of holes by adding or removing ions. However , when we use a field effect gate, we see the opposite effect. Adding or removing holes always causes an increase in conductivity!
Accumulating unbalanced government power
The researchers were able to trace these unexpected effects to the ‘Coulomb gap’, a well-known, albeit rare, feature of semiconductors. Interestingly, this effect disappears at room temperature and the expected pattern is restored.
“Coulomb gaps are known to be difficult to detect in electrical measurements, because they are only visible when the material cannot find its stable shape,” Jacobs added. On the other hand, we were able to see these effects at temperatures much higher than expected, only about -30°C.
It turns out that in our matter, ions freeze; this can happen at relatively high temperatures,” said Ren. “If we add or remove electrons when the ions are frozen, the material is in an unstable state. The ions can choose to rearrange and stabilize the system, but they can’t because they are frozen. This allows us to see the Coulomb gap.”
Often, there is a trade-off between thermoelectric power generation and conductivity, one increases while the other decreases. However, due to the Coulomb gap and unbalanced effects, both can be added together, which means that the performance can be improved. The only limitation is that the field effect gate now only affects the surface of the material. If the density of the material is affected, it can increase the strength and conductivity to a large extent.
Although the team still has a ways to go, the research paper offers a clear way to improve the performance of organic semiconductors. With exciting prospects in the field of energy, the team left the door to further research of this property. “Transport in these non-equilibrium regions has also proven to be a promising route for better organic thermoelectric devices,” said Tjhe.
Reference: “Non-equilibrium transport in polymer mixed ionic–electronic conductors at ultrahigh charge densities” by Dionisius HL Tjhe, Xinglong Ren, Ian E. Jacobs, Gabriele D’Avino, Tarig BE Mustafa, Thomas G. Marsh, Lu Zhang, Yao Fu, Ahmed E. Mansour, Andreas Opitz, Yuxuan Huang, Wenjin Zhu, Ahmet Hamdi Unal, Sebastiaan Hoek, Vincent Lemaur, Claudio Quarti, Qiao He, Jin-Kyun Lee, Iain McCulloch, Martin Heeney, Norbert Koch, Clare P. Gray , David Beljonne, Simone Fratini and Henning Sirringhaus, 26 July 2024, Natural Things.
DOI: 10.1038/s41563-024-01953-6
#Solving #Doping #Problem #Physicists #Discover #Ways #Improve #Organic #Semiconductors